EXECUTIVE SUMMARY
The purpose of this study was to conduct A pilot clinical trial on the effects of a new nutraceutical formula from Bode Pro, Inc. on human mitochondria at the cellular level in order to help develop a clinical proof-of-concept trial. A number of components in the nutraceutical formula TEN are recognized to support mitochondrial activity.
After 2 hours, cells treated with an aqueous portion of the TEN nutraceutical formula had dramatically increased mitochondrial mass. The timeframe (2 hours) is favorable for future human clinical research that will include the mitochondrial mass assay.
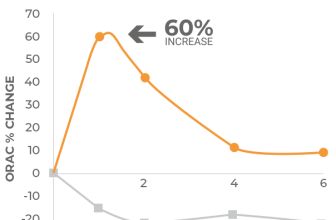
Pilot research was also conducted on TEN’s total antioxidant capacity (TAC) in order to determine the antioxidant levels present in an aqueous version of the TEN formula. This is pertinent to the antioxidants that are likely to be accessible in future clinical investigations after human ingestion.
RESULTS
2 KEY STUDY OUTCOMES:
1. Mitochondrial Mass Per Cell (MMPC): This is defined as a compound’s ability to increase the amount of mitochondria in human leukocytes (white blood cells).
2. Total Antioxidant Capacity (TAC): The ability of a chemical to protect human leukocytes from free radical damage.
ENDPOINTS:
1. Mitochondrial Mass Per Cell (MMPC): Increases in MMPC were observed 2 hours after cells were treated with the TEN mixture (see graphs below).
• In three separate white blood cell types, the 0.125 g/L dose of the TEN formula resulted in an increase in MMPC of 80-140 percent.
This demonstrates the TEN formula’s relative potency, which bodes well for future clinical investigations in which mitochondrial mass per cell will be measured in blood samples from human participants before and after they consume the TEN formula.